About the project
This project uses data from the National Integrated Health Services Information (NIHSI) (version 2.0) – a linked administrative data set held by the AIHW. The data sets used in this project were:
- admitted patient care data from the National Hospital Morbidity Database
- deaths data from the National Death Index
- prescription medications data from the Pharmaceutical Benefits Scheme (PBS) and the Repatriation Pharmaceutical Benefits Scheme (RPBS)
- data on use of Medicare-subsidised health services from the Medicare Benefits Schedule (MBS).
The NIHSI holds data from New South Wales, Victoria, Queensland, South Australia, Tasmania and the Australian Capital Territory from 2010–11 to 2019–20 for the above-mentioned datasets. In most states, the admitted patient care data are limited to only public hospitals. Additional information about NIHSI can be found on the AIHW website.
For a discussion of the limitations see Limitations of the data.
Cohort
The cohort comprised 35,784 people, aged between 25 and 84, who had an acute coronary syndrome (ACS) hospitalisation with an acute care type between 1 July 2016 and 30 June 2017 and were alive at the point of discharge. See Table S1 in the supplementary data tables for details of cohort selection.
A diagnosis of acute coronary syndrome was defined in the admitted patient care data using the principal diagnosis field (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification (ICD-10-AM): I21.0-I21.4. I21.9, I20.0).
Acute care type indicates hospital care in which the intent is to perform surgery, diagnostic or therapeutic procedures in the treatment of illness or injury. It excludes rehabilitation or palliative care.
What is acute coronary syndrome?
Acute coronary syndrome (ACS) is a term used to describe a continuum of acute coronary artery diseases (including heart attacks and unstable angina). These conditions are sudden, severe and life-threatening events.
A heart attack (or acute myocardial infarction) is a life-threatening event commonly where a blocked blood vessel threatens to damage the heart muscle. Clinically, a heart attack is often categorised based on the pattern that appears on an electrocardiogram (ECG), a diagnostic tool that measures and records the heart’s electrical activity.
- STEMI, or ST segment elevation myocardial infarction, is so named because the ‘ST segment’ on the ECG appears elevated. STEMI is a type of heart attack almost always caused by a complete blockage to a major coronary artery. This is the most easily diagnosed subtype of ACS.
- NSTEMI, or non-ST segment elevation myocardial infarction, is a type of heart attack in which an artery is frequently partially blocked. This severely reduces blood flow. Unlike STEMI, the ‘ST segment’ on the ECG is not elevated. There may or may not be other changes on the ECG. The diagnosis of a NSTEMI involves identification of elevated troponin levels through assays in a person with a clinical presentation consistent with a heart attack.
- Unspecified myocardial infarction is a clinical code within hospital data which does not specify the myocardial infarction diagnosis as either STEMI or NSTEMI.
Angina is a chronic condition in which intermittent episodes of chest pain can occur when the heart has a temporary deficiency in blood supply. Unstable angina can be dangerous due to the changing severity in transient coronary narrowing.
Reference period
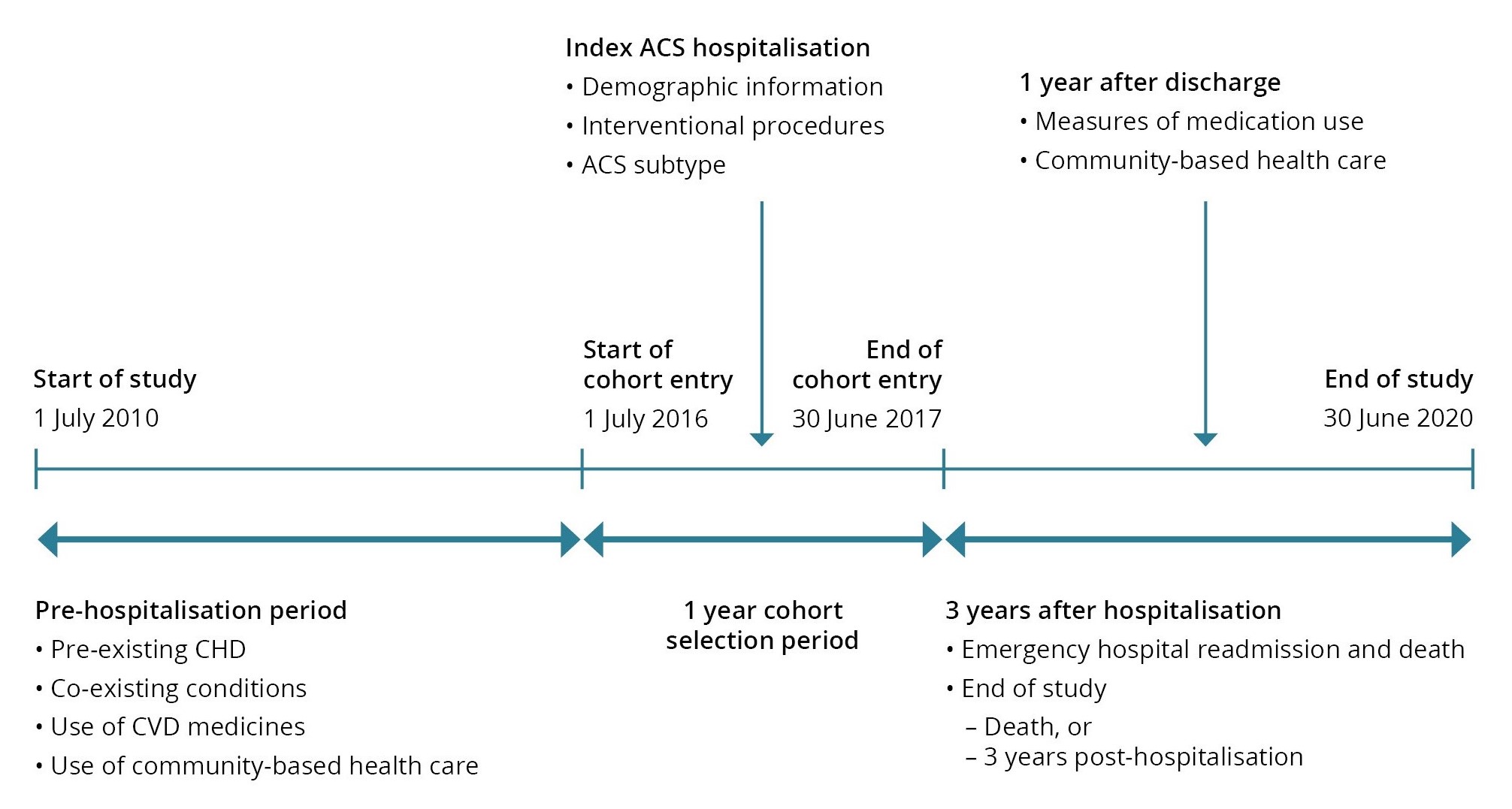
Comorbidities, including prior hospitalisation for coronary heart disease (CHD), and use of in-scope cardiovascular disease (CVD) medicines were identified in the period 1 July 2010 (start of the data set) to the index hospitalisation in the 2016–17 financial year.
Demographic information, ACS subtype and interventional procedures were identified from the index hospitalisation record.
The follow-up period started on the day after discharge and ended at the end of the study period (3 years post-hospitalisation) or death – whichever occurred first. The first year of the follow-up period was used to examine treatment pathways in the cohort, including use of medications. Community-based health care, specifically visiting a cardiologist, was measure in the first year using MBS data. Health outcomes (emergency CVD readmissions and death) were measured for the 3 years.
See the supplementary data tables for:
- the classification of acute coronary syndrome subtypes and comorbidities (Table S6)
- analysis variables and descriptions (Table S8).
See Figure 1 for an illustration of the reference period.
Figure 1: Reference period
Key variables
Medication use
Initiation: defined as the first PBS dispensing record for the relevant medication following hospital discharge. This project captures initiation to the 4 classes of guideline recommended medications within 40 days of discharge from the index hospitalisation.
The time point of 40 days post discharge was used to measure post-hospitalisation initiation (the filling of a medication prescription) to account for up to one month’s supply a person may already have from before they went to hospital, plus up to 7 days of medication which may be supplied when leaving hospital in some Australian states.
Note: Aspirin was not included in this study, for details see Limitations of the data.
Persistence: the continuation of prescribed treatments for the recommended period. In this project, a person was classified as being no longer persistent (discontinued) when there was a gap in medication supply of 60 days or more for any of the 4 medication classes. As with initiation, persistence to all 4 medication classes was reported to capture alignment to Guideline recommendations. Time spent in public hospitals was excluded from this measure as the supply of medications in this setting would not be captured in the PBS data. Persistence was measured for each medication class from the first supply until one year post hospitalisation. The first supply could be at any time in the first year (unlike initiation in which a 40-day window was used), and at least 2 supplies were required. Persistence was not measured for people who died within 1 year of discharge.
For more information about how these measures are derived see Medication use for secondary prevention after coronary heart disease hospitalisations: patient pathways using linked data – technical report.
For capture of ATC codes for medication classes see Table S7 in the supplementary data tables.
Interventional CVD procedures
Intervention procedures measured:
Coronary artery bypass grafting (CABG): surgical procedure using blood vessel grafts to bypass blockages in the coronary arteries and restore adequate blood flow to the heart muscle.
Percutaneous coronary intervention (PCI): restore blood flow to blocked coronary arteries. There are 2 types: coronary angioplasty without stent, and coronary stenting.
For consistency, this same period (40 days) used for initiation was applied to capture intervention procedures that occurred soon after the index hospitalisation.
Capture of interventions that occurred during the 40 days after index hospitalisation will be an underestimate, due to no availability of private hospital data for some states.
Comorbidities
Comorbidity information was obtained for the following conditions in the admitted patient care data only: Pre-existing CHD, Congestive heart failure, Chronic obstructive pulmonary disease, Kidney failure, Peripheral vascular disease, Cancer, Diabetes, Hypertension, and Cerebrovascular disease. Therefore, it is likely an underestimate of comorbidities in the cohort.
Health outcomes
CVD readmission: emergency readmission to hospital with an urgent care type and principal diagnosis of CVD (ICD-10-AM: I00-I99).
Major adverse cardiac events (MACE): emergency hospital readmission (principal diagnosis) or death (underlying cause) due to ACS, stroke, or heart failure (ICD-10 and ICD-10-AM: I20.0 I21, I50, I60-I64).
CVD death: death with an underlying cause of CVD (ICD-10: I00-I99).
All cause death: Death from any cause.
For variable descriptions see Table S8 in the supplementary data tables.


