Example 3: Health service use and prescriptions dispensed
On this page:
The following sections demonstrate how the COVID‑19 Register could be used to examine health service use and prescriptions dispensed before and after a COVID‑19 diagnosis using claims data from the Medicare Benefits Schedule (MBS) and the Pharmaceutical Benefits Scheme (PBS). The COVID‑19 Register will make it possible to examine patient pathways following a COVID‑19 diagnosis to determine if there are changes to health service use.
In the analysis cohort used in these sections, people with more than one COVID‑19 diagnosis (192 people) were excluded to ensure that patterns observed were only related to their first diagnosis. Future analysis could explore how reinfections also impact service usage including in relation to other protective factors such as vaccination.
Definition of Medicare Benefits Schedule and Pharmaceutical Benefits Scheme services
The Medicare Benefits Scheme provides a subsidy for services listed in the Australian Government Department of Health and Aged Care’s Medicare Benefits Schedule (MBS), for all Australian residents and certain categories of visitors to Australia.
For more details of the services covered under the MBS see Technical notes.
The Pharmaceutical Benefits Scheme (PBS) is a program where the Australian Government subsidises the cost of a wide range of prescription medicines through 2 separate schemes:
- the PBS
- the Repatriation Pharmaceutical Benefits Scheme (RPBS).
Claims for reimbursement for the supply of PBS- or RPBS-subsidised medicines are submitted by pharmacies to Services Australia for processing and are provided to the Australian Government Department of Health and Aged Care. Subsidies for prescription medicines are available to all Australian residents who hold a current Medicare card, and overseas visitors from countries with which Australia has a Reciprocal Health Care Agreement. Patients pay a contribution to the cost of the medicine (co-payment), and the Australian Government covers the remaining cost.
For more details of the medicines covered under the PBS see Technical notes.
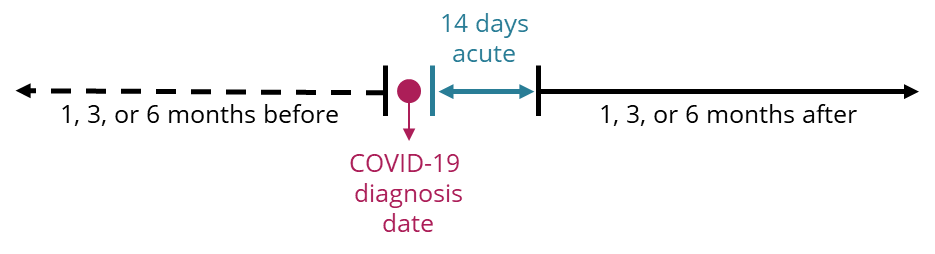
To focus on the post-acute impact, the example analysis considers health services accessed and prescriptions dispensed 1, 3 or 6 months before or after a COVID-19 diagnosis. However, when considering usage patterns after a COVID-19 diagnosis, usage patterns in the 14 days (acute phase) after their COVID‑19 diagnosis date is not considered, as shown in Figure 6. Future analysis could just explore this period, for researchers interested in health service use during the acute phase of a COVID‑19 diagnosis.
Figure 6: Determining health services accessed and prescriptions dispensed before and after COVID‑19 diagnosis for the linked analysis cohort
Medicare Benefits Schedule service use before and after a COVID‑19 diagnosis
Broad type of service use before and after a COVID‑19 diagnosis
The aim of this section was to demonstrate how researchers could use the COVID‑19 Register to investigate health service use by broad type of Medicare Benefits Schedule (MBS) services before and after a COVID‑19 diagnosis. MBS-subsidised services are reported using the broad type of service (BTOS) classification, whereby each MBS item is allocated to a BTOS category (see Technical notes for more detail). For the purpose of analysing health service use, the example analysis also excluded services related to COVID‑19 vaccine support, receiving a COVID‑19 vaccine and COVID‑19 PCR testing as these are services that arise in response to the pandemic, which are driven by considerations/ factors outside the health impacts of the disease itself.
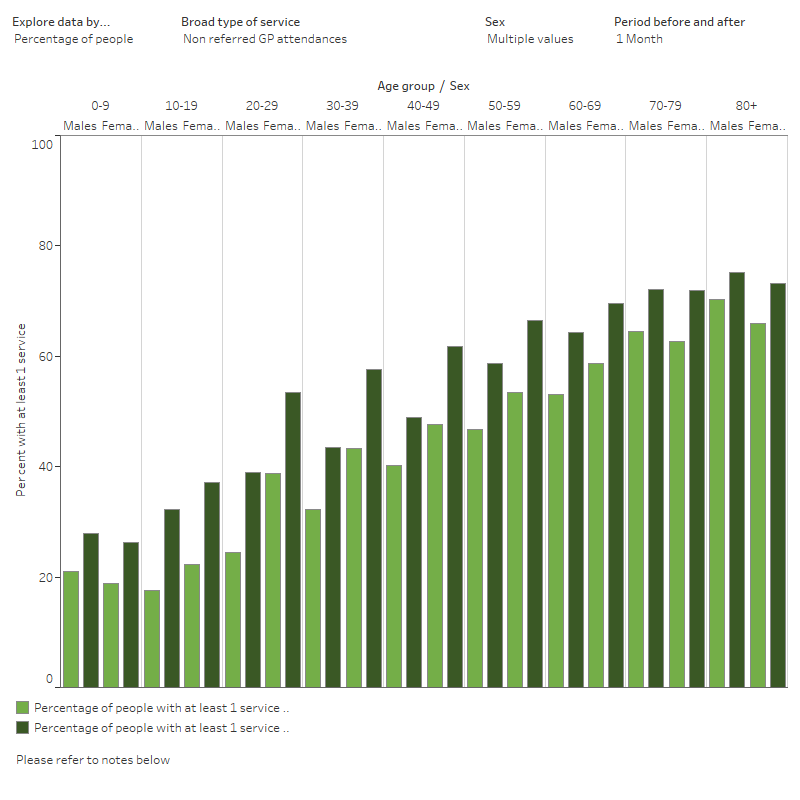
Figure 7 shows that for both males, females and total persons across all age groups, a higher percentage of the analysis cohort had non-referred attendances (GP visits) at 1 and 3 months following their COVID‑19 diagnosis when compared with before diagnosis. At 6 months, this difference was less apparent across the older age groups. This could be explained by a range of factors that were not explored in this preliminary example analysis. This example demonstrates the ability to look at health service usage before and after COVID‑19 by demographic factors. Future analysis could explore these results by remoteness, cultural and linguistic diversity or by socioeconomic group.
The interactive data visualisation (Figure 7) presents statistics on MBS services accessed. To customise the visualisation, use any of the 4 drop-down menus to explore data by measure, broad type of service, sex and period before and after (COVID-19 diagnosis). Access the Data table using the tab. There are 4 drop-down menus where the data can be filtered by measure, broad type of service, sex and the period before and after (COVID‑19 diagnosis).
Figure 7: MBS services accessed by the analysis cohort in 1, 3 and 6 months before and after a COVID‑19 diagnosis, by broad type of MBS service (aggregated), age group and sex
Chart shows the percentage of people in the linked analysis cohort with at least 1 MBS service 1, 3, or 6 months before and after a COVID‑19 diagnosis, by broad type of service and 10-year age groups.
Notes:
- Excludes services related to COVID‑19 vaccine support, receiving a COVID‑19 vaccine, and PCR tests (from the ‘Pathology tests’ category).
- Excludes people who had multiple COVID‑19 diagnosis and who died (of any cause) within 6 months of their diagnosis.
- Refer to Technical notes for details on what is included under each BTOS category.
- Age group is based on age as at 31 December 2021.
Source: AIHW analysis of COVID‑19 Register (version 1)
In this section, COVID‑19 PCR tests were excluded from the ‘Pathology tests’ category, to observe if this had any impact to the findings. However COVID‑19 PCR tests were unable to be excluded from the ‘Pathology collection items – Pathology Patient Episode Initiation’ category, and these results should be interpreted with this in mind. When excluded, there was no longer a decrease in the percentage of the cohort with a pathology test after COVID‑19, and for most age groups, there was a slight increase in the percentage who had a pathology test after a diagnosis in the 1, 3, and 6 months following a diagnosis.
Future analyses will need to consider confounding factors and seasonality of health service use, and comparisons made with the general population and those without a COVID‑19 diagnosis.
Prescriptions dispensed before and after a COVID‑19 diagnosis
This analysis demonstrates the use of PBS claims data to look at prescriptions dispensed 1, 3 and 6 months before and after a COVID‑19 diagnosis in the analysis cohort. As COVID‑19 is predominantly a respiratory infection, the impact of COVID‑19 on respiratory prescriptions (using the PBS classification ‘Drugs for Obstructive Airway Diseases’ or ATC code R03) was also explored in this example analysis. Subsequent analysis on future versions of the data could look at other types of medications, such as cardiovascular disease and mental health scripts.
For this example, prescriptions with a drug type ‘prescriber’s bag’ (or more commonly known as doctor’s bag) have been excluded in this example as they are a specific range of medicines usually pre-ordered by a doctor for emergency use.
Percentage of the cohort with a PBS script before and after a COVID‑19 diagnosis
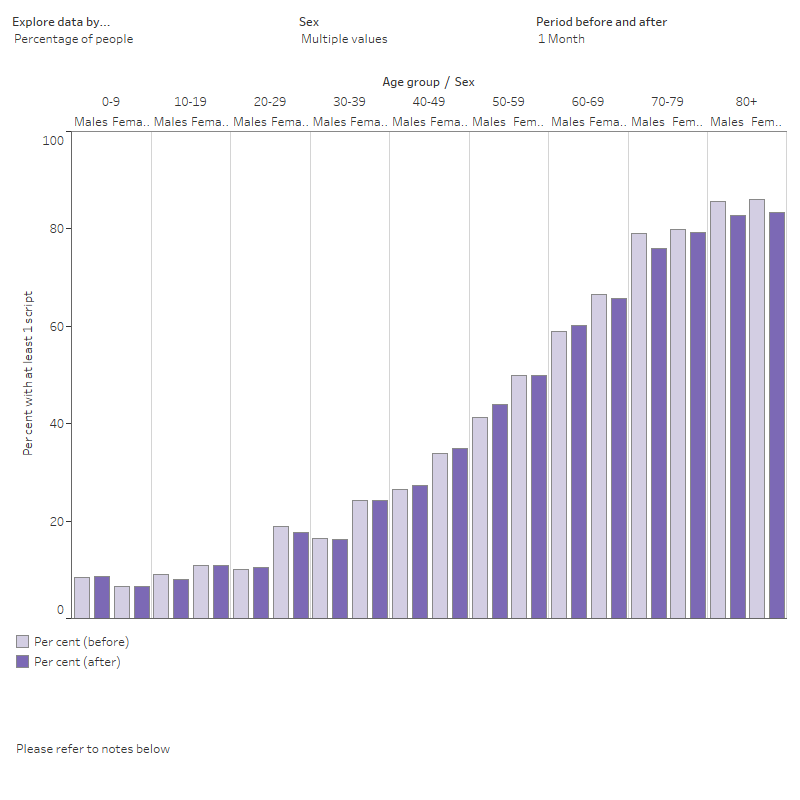
The aim of this section is to demonstrate one method of looking at prescription supply patterns for people in the COVID‑19 Register. It explores the percentage of the analysis cohort with a PBS script (all scripts and respiratory medicines only) dispensed 1, 3 and 6 months before and after a COVID‑19 diagnosis (Figure 8).
Figure 8 also presents the percentage of the cohort with at least 1 respiratory medicine dispensed 1, 3 and 6 months before and after a COVID‑19 diagnosis. There were mostly no major differences before and after COVID‑19, except for those aged over 70 at 3- and 6-months post diagnosis. This could be explained by a range of factors that were not explored in this preliminary example analysis.
Analyses in the future on medications dispensed will need to consider confounding factors and seasonality of prescription rates, and comparisons made with the general population and unlinked data sources.
The interactive data visualisation (Figure 8) presents statistics on PBS scripts (including R03 scripts) dispensed. The visualisation can be customised according to selection of the 3 drop-down menus for measure, sex and period before and after (COVID-19 diagnosis). Access the Data table using the tab. There are 3 drop-down menus where the data can be filtered by measure, sex and period before and after (COVID-19 diagnosis).
Figure 8: PBS scripts dispensed per person 1, 3 and 6 months before and after a COVID‑19 diagnosis in the analysis cohort, by medication type, age group and sex
Chart show the percentage of people in the linked analysis cohort with at least one PBS or R03 script 1, 3 or 6 months before and after a COVID-19 diagnosis, by 10-year age groups and sex.
Notes:
- Excludes people who had multiple COVID‑19 diagnosis and who died (of any cause) within 6 months of their diagnosis.
- R03 refers to drugs for obstructive airway diseases.
- Age group is based on age as at 31 December 2021.
Source: AIHW analysis of COVID‑19 Register (version 1)


