Introduction
On this page:
Using the COVID‑19 Register to examine the impact of COVID‑19 on health outcomes and service use
There is still little known about the health and health system effects of COVID‑19, the impact of reinfections and how COVID‑19 interacts with other chronic diseases. Long COVID specifically is a complex, multi-system illness with the potential for a substantial impact on society, from increased health care costs to economic and productivity losses. Symptoms may persist for weeks or months following acute SARS-CoV-2 infection, come and go over time, or manifest as new onset chronic conditions, such as heart disease, diabetes, kidney disease and neurological conditions (AIHW 2022a).
Large health databases that link COVID‑19 case information to administrative datasets have been used in international studies to assess the impact of COVID‑19 (AIHW 2022a). The Australian Institute of Health and Welfare's (AIHW) literature review on long COVID found that studies from the US, have identified an increased risk of a range of chronic outcomes, including cardiovascular disease, metabolic disorders, and mental and neurological complaints up to 12 months following infection (AIHW 2022a).
Linked data has also been used to assess vaccine and antiviral effectiveness internationally (International Vaccine Access Center 2023; Wai et al. 2023). Australia’s experience of COVID‑19 was very different to other countries and we have had differing eligibility for vaccines and treatments. Additionally, compared to other countries, Australia had a delay in widespread virus circulation, and was able to establish the assessment of some interventions before transmission took off. It is therefore imperative that these international studies are corroborated with local information.
The COVID‑19 Register is the first national source of linked COVID‑19 case information that researchers can start exploring these questions in an Australian setting.
Aim of this report
This report aimed to use a cohort of people who tested positive for COVID‑19 from the newly established COVID‑19 Register to demonstrate how the data could be used by looking at some preliminary health service use patterns before and after a COVID‑19 diagnosis, using linked Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS) data.
Demographic information and health outcome data has also been included in this report as other analysis examples and to give context to the health service use sections.
Scope of the cohort used for demonstration of analytical approaches
The COVID‑19 Register is being built iteratively, and at the time of this analysis, data supplied by 5 jurisdictions (based on version 1 of the COVID‑19 Register) were included for the examples used in this report: New South Wales, South Australia, Tasmania, Australian Capital Territory and the Northern Territory. Further information about the establishment of the COVID‑19 Register, linkage methods and more information for researchers wanting to use this data can be found at: COVID‑19 register and linked data set.
The precise reference period of notifications varied by jurisdiction for the COVID‑19 cases available in version 1 of the COVID‑19 Register (see Technical notes). Figure 1 details how the analysis cohort used in the examples in this report was derived, and exclusions that were applied.
To allow for sufficient heath service use information before and after a COVID‑19 diagnosis, the examples presented in this report focus on people whose first COVID‑19 notification had a diagnosis date between 16 June and 14 December 2021, when the Delta variant was dominant. This time period was also chosen for the analysis cohort as the detection of cases was high using widely available Nucleic Acid Testing (also knows as PCR tests). The use of Rapid Antigen Tests (RATs) and self-testing/ reporting was relatively low during this time and only became more common during the Omicron wave in 2022. It also aligns with reporting of variants in the Communicable Diseases Intelligence COVID‑19 Epidemiology reports.
This report is a preliminary exploration of the data to demonstrate how it could be used. Given it is just a demonstration, potential confounding effects that may also contribute to changes in service use, including vaccination and prior infection have not been accounted for and the findings should not be interpreted as a true reflection of the impact of COVID‑19 in Australia. Analysis of the differential impacts of COVID‑19 on a range of priority populations, such as aged care residents or people with a disability, is crucial. Although it is out of scope of this report, it will be another important type of analysis made possible by this data set.
The COVID‑19 Register uses probabilistic linkage to link COVID‑19 cases to administrative data sets and while the linkage rates and quality is generally quite good, there are limitations in how well data linkage can capture a truly representative population.
For further information on how the cohort was created, data linkage methods and definitions used in the report, refer to the Technical notes.
Figure 1: Creation of the COVID‑19 analysis cohort used in the demonstration examples
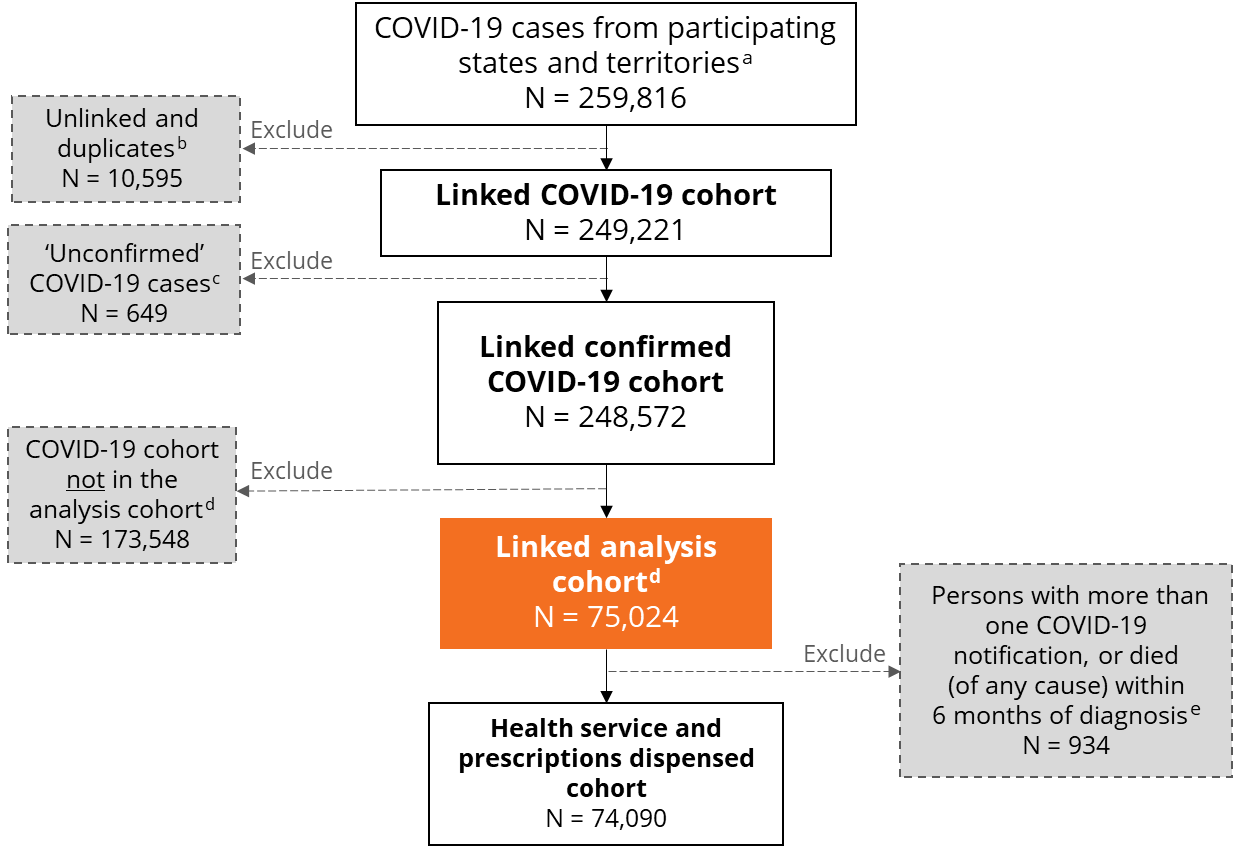
Notes:
- N refers to number of persons rather than total cases. Participating states and territories for COVID‑19 Register version 1 are NSW, SA, Tasmania, ACT and NT. Total number of cases is 265,561.
- Refers to records which cannot be matched to any of the datasets in the COVID‑19 Register. Refer to web report COVID‑19 linked data set: Linkage results for details on how the data was linked.
- The variable ‘confirmation status’ from the National Notifiable Diseases Surveillance System (NNDSS) was used to exclude cases from the cohort which did not fall into our definition of eligible cases. For more details on how this was done, refer to the Technical notes.
- The analysis cohort refers to people whose first COVID‑19 notification had a diagnosis date between 16 June and 14 December 2021, when the Delta variant was dominant.
- Includes people whose date of death was before their COVID‑19 diagnosis date.
References
AIHW (Australian Institute of Health and Welfare) (2022a) Long COVID in Australia, AIHW, Australian Government, accessed 25 May 2023.
International Vaccine Access Center (IVAC) (2023) Effectiveness studies, International Vaccine Access Centre website, accessed 15 June 2023.
Wai AKC, Chan CY, Cheung AWL, Wang K, Chan SCL, Lee TTL, Luk LFY, Yip ETF, Ho JWK, Tsui OWK, Cheung KWY, Lee S, Tong CK, Yamamoto T, Rainer TH and Wong ElY (2023) ‘Association between Molnupiravir and Nirmatrelvir – Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19’ The Lancet Regional Health Western Pacific, 30:100602. DOI:10.1016/j.lanwpc.2022.100602.


